
This is a patient* with advanced or metastatic gastric or GEJ adenocarcinoma – someone who knows their situation, but is not surrendering to it.
- Presents with difficulty swallowing
- No prior surgery
- Unresectable stage IV gastric or GEJ adenocarcinoma
- FOLFOX 1L; oxaliplatin dropped after 4 cycles due to low-grade neuropathy
- Disease progressed after 6 months
- Current scan shows metastases in distant lymph nodes and liver
- ECOG PS 1
* Hypothetical patient example
Can you picture one of your patients? Someone who is determined and counting on you for answers?
FOLFOX = fluorouracil and oxaliplatin, ECOG = Eastern Cooperative Oncology Group, PS = Performance Status
CYRAMZA as a single agent, or in combination with paclitaxel, is indicated for the treatment of patients with advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma with disease progression on or after prior fluoropyrimidine- or platinum-containing chemotherapy.
CYRAMZA + paclitaxel: The most frequently prescribed regimen in 2L†
CYRAMZA + Paclitaxel Is Proven to Extend Survival, Delay Progression, and Improve Response Rate2 (versus placebo + paclitaxel)
† Patients age 18 and older identified from a retrospective analysis of electronic medical record (EMR) data (Flatiron Health Data, New York, NY) who were confirmed to have advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma and who started first-line therapy between January 1, 2015 and February 28, 2019 were included. Patients were confirmed to have received these agents in an EMR system.1

‡ The percentage of deaths at the time of analysis was 78% (256 patients) and 78% (260 patients) in the CYRAMZA plus paclitaxel and placebo plus paclitaxel treatment arms, respectively.2
The following three figures present efficacy data for the RAINBOW trial.
Overall survival (OS) was a major outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel (n equals 330), median OS was 9.6 months with a 95 percent confidence interval of 8.5 to 10.8 months. With placebo plus paclitaxel (n equals 335), median OS was 7.4 months with a 95 percent confidence interval of 6.3 to 8.4 months. The hazard ratio was 0.81 with a 95 percent confidence interval of 0.68 to 0.96 and a P value of 0.017. The percentage of deaths at the time of the analysis was 78 percent (256 patients) in the CYRAMZA plus paclitaxel treatment arm and 78 percent (260 patients) in the placebo plus paclitaxel treatment arm of the trial.

‖ The percentage of events at the time of analysis was 85% (279 patients) and 88% (296 patients) in the CYRAMZA plus paclitaxel and placebo plus paclitaxel treatment arms, respectively.2
56 of 279 events in CYRAMZA-treated patients and 55 of 296 events in placebo-treated patients were deaths.
Progression-free survival (PFS) was a supportive outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel (n equals 330), median PFS was 4.4 months with a 95 percent confidence interval of 4.2 to 5.3 months. With placebo plus paclitaxel (n equals 335), median PFS was 2.9 months with a 95 percent confidence interval of 2.8 to 3.0 months. The hazard ratio was 0.64 with a 95 percent confidence interval of 0.54 to 0.75 and a P value of less than 0.001. The percentage of events at the time of analysis was 85 percent (279 patients) in the CYRAMZA plus paclitaxel treatment arm and 88 percent (296 patients) in the placebo plus paclitaxel treatment arm. In CYRAMZA-treated patients, 56 of 279 events were deaths. In the placebo-treated patients, 55 of 296 events were deaths.

¶ Overall response rate was defined as complete plus partial response. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.5
ORR included 2 complete responses in CYRAMZA-treated patients and 1 complete response in placebo-treated patients.
Overall response rate (ORR) was a supportive outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel (n equals 330), ORR was 28 percent of patients with a 95 percent confidence interval of 23 to 33 percent. With placebo plus paclitaxel (n equals 335), ORR was 16 percent of patients with a 95 percent confidence interval of 13 to 20 percent. The P value was less than 0.001. ORR was defined as complete response (CR) plus partial response (PR). Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. ORR included 2 complete responses in CYRAMZA-treated patients and 1 complete response in placebo-treated patients.
SELECT IMPORTANT SAFETY INFORMATION
The labeling for CYRAMZA contains warnings and precautions for hemorrhage and GI hemorrhage, including severe and sometimes fatal events; gastrointestinal (GI) perforations, a potentially fatal event; impaired wound healing; arterial thromboembolic events (ATEs), including serious and sometimes fatal events; hypertension; infusionrelated reactions (IRR), including severe and sometimes fatal events; worsening of pre-existing hepatic impairment; posterior reversible encephalopathy syndrome (PRES), including fatal events; proteinuria including nephrotic syndrome; thyroid dysfunction; and embryo-fetal toxicity. CYRAMZA should be permanently discontinued in patients who experience severe bleeding, a GI perforation, an ATE, uncontrolled hypertension, severe IRR, PRES, or urine protein >3 grams/24 h or nephrotic syndrome.
In RAINBOW, the most common adverse reactions (all Grades) observed in patients treated with CYRAMZA with paclitaxel at a rate of ≥30% and ≥2% higher than placebo with paclitaxel were fatigue/asthenia (57% vs 44%), neutropenia (54% vs 31%), diarrhea (32% vs 23%), and epistaxis (31% vs 7%). The most common serious adverse reactions with CYRAMZA with paclitaxel were neutropenia (3.7%) and febrile neutropenia (2.4%); 19% of patients treated with CYRAMZA with paclitaxel received granulocyte colony-stimulating factors.
RAINBOW Trial Design (N=665)2,6
The phase III RAINBOW trial evaluated the efficacy and safety of CYRAMZA plus paclitaxel vs placebo plus paclitaxel in patients with locally advanced or metastatic gastric or GE junction adenocarcinoma with disease progression on or after prior fluoropyrimidine- and platinum-containing chemotherapy. Major efficacy outcome measure was overall survival. Supportive efficacy outcome measures were progression-free survival and overall response rate. All patients were Eastern Cooperative Oncology Group performance status 0 or 1. Prior to enrollment, 97% of patients had progressed during treatment or within 4 months after the last dose of first-line chemotherapy for metastatic disease. Twenty-five percent of patients had received anthracycline in combination with platinum/fluoropyrimidine therapy, while 75% did not. Patients were randomized 1:1 to CYRAMZA 8 mg/kg (n=330) or placebo (n=335) every 2 weeks (on days 1 and 15) of each 28-day cycle. Patients in both arms received paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 28-day cycle.
Ramucirumab + paclitaxel: The only FDA-approved combination regimen for the treatment of advanced or metastatic gastric or GEJ adenocarcinoma in the second-line setting and a Category 1 recommendation within the NCCN Guidelines®2-4
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommendations for ramucirumab (CYRAMZA)
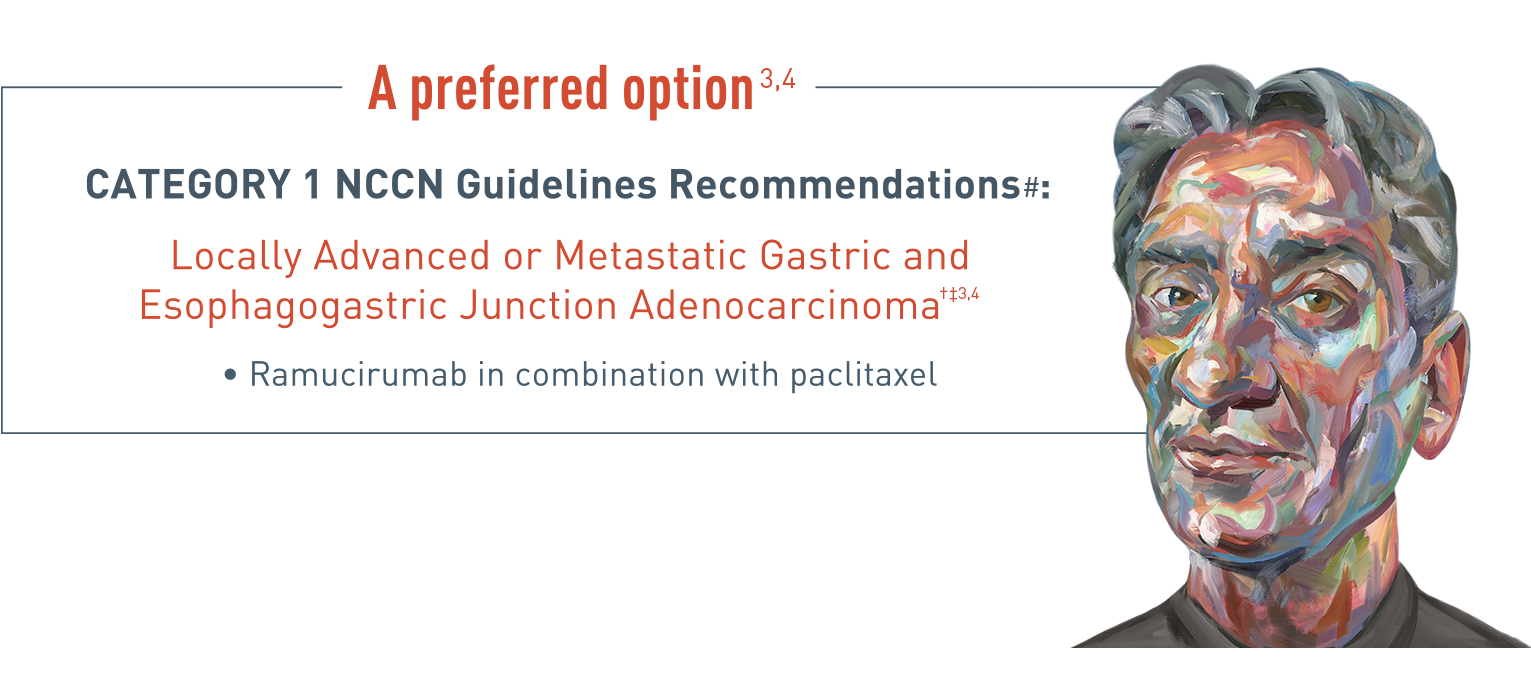
A preferred option for treatment of locally advanced or metastatic gastric and esophagogastric junction adenocarcinoma.
Category 1 National Comprehensive Cancer Network (NCCN) guidelines recommendations for locally advanced or metastatic gastric and esophagogastric junction adenocarcinoma include ramucirumab in combination with paclitaxel.
Recommendations are referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Gastric Cancer v5.2021 and Esophageal and Esophagogastric Junction Cancers v4.2021. NCCN guidelines for Gastric Cancer v5.2021 recommend ramucirumab (CYRAMZA) in combination with paclitaxel as a preferred second-line treatment option for locally advanced or metastatic gastric adenocarcinoma. NCCN Guidelines for Esophageal and Esophagogastric Junction (EGJ) Cancers v4.2021 recommend ramucirumab (CYRAMZA) in combination with paclitaxel as a preferred second-line treatment option for locally advanced metastatic EGJ adenocarcinoma.
CATEGORY 1: Based upon high-level evidence, there is uniform National Comprehensive Cancer Network® (NCCN®) consensus that the intervention is appropriate3,4
SELECT IMPORTANT SAFETY INFORMATION
Hemorrhage
- CYRAMZA increased the risk of hemorrhage and gastrointestinal hemorrhage, including Grade ≥3 hemorrhagic events. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade hemorrhage ranged from 13- 55%. Grade 3-5 hemorrhage incidence ranged from 2-5%.
- Patients with gastric cancer receiving nonsteroidal anti-inflammatory drugs (NSAIDs) were excluded from enrollment in REGARD and RAINBOW; therefore, the risk of gastric hemorrhage in CYRAMZA-treated patients with gastric tumors receiving NSAIDs is unknown.
- Permanently discontinue CYRAMZA in patients who experience severe (Grade 3 or 4) bleeding.
§ Median.
# Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer v2.2020 and Esophageal and Esophagogastric Junction Cancers v2.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed June 18, 2020. To view the most recent and complete version of the guideline, go online to NCCN.org.
** NCCN Guidelines® for Gastric Cancer v2.2020 recommend ramucirumab (CYRAMZA) in combination with paclitaxel as a preferred second-line treatment option for locally advanced or metastatic gastric adenocarcinoma.3
†† NCCN Guidelines® for Esophageal and Esophagogastric Junction (EGJ) Cancers v2.2020 recommend ramucirumab (CYRAMZA) in combination with paclitaxel as a preferred second-line treatment option for locally advanced or metastatic EGJ adenocarcinoma.3
The National Comprehensive Cancer Network makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
1L=first line; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; FOLFOX=folinic acid, fluorouracil, and oxaliplatin; GEJ=gastroesophageal junction; HR=hazard ratio; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PS=performance status.
References
- Data on file, Eli Lilly and Company. ONC20170210d.
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer v2.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed June 18th 2020. To view the most recent and complete version of the guidelines, go online to https://www.nccn.org.
- Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Esophageal and Esophagogastric Junction Cancers v2.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed June 18th 2020. To view the most recent and complete version of the guidelines, go online to https://www.nccn.org.
- Wilke H, Muro K, Van Cutsem E, et al; for the RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235.
- Data on file, Eli Lilly and Company. ONC09302014b.
Second-line treatment trends
Learn about how the second-line treatment landscape has evolved